The reaction coordinate of a bacterial GH47 α-mannosidase: a combined quantum mechanical and structural approach.
The reaction coordinate of a bacterial GH47 α-mannosidase: a combined quantum mechanical and structural approach
A. J. Thompson, J. Dabin, J. Iglesias-Fernández, A. Ardèvol, Z. Dinev, S. J. Williams, O. Bande, A. Siriwardena, C. Moreland, T.-C. Hu, D. K. Smith, H. J. Gilbert, C. Rovira*, G. J. Davies*.
Angew. Chem. Int. Ed. 51 (2012) 10997-11001. Selected as Very Important Paper (VIP, top 5% of the journal). Inside back cover.
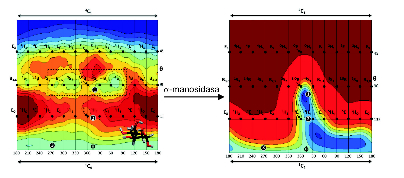
Conformational space reduction of a mannose unit by the α-manosidase enzyme,
as well as its conformational itinerary during the chemical reaction.
α-manosidase enzymes are responsible of trimming and/or remodeling certain carbohydrates that are covalently attached to proteins. Alteration, excess and deficiency of these carbohydrates lead to diseases such as some tumors or the so-called lysosomal diseases. Starting from the structure of the carbohydrate-enzyme complex, determined at atomic resolution, and using ab initio molecular dynamics-based methods (in particular, QM/MM metadynamics) we demonstrated that the enzyme drastically alters the conformational landscape of the carbohydrate with respect to the one of the isolated carbohydrate. Interestingly, the conformations that “survive” are the ones that the carbohydrate adopts during the catalytic reaction. Therefore, the enzyme imposes the conformations that favor catalysis.
