A Fullerene-Carbene Adduct as a Crystalline Molecular Rotor: Remarkable Behavior of a Spherically-Shaped Rotator
A Fullerene-Carbene Adduct as a Crystalline Molecular Rotor: Remarkable Behavior of a Spherically-Shaped Rotator
E. Lorbach, E. Maverick, A. Carreras, P. Alemany, G. Wu, M.A. Garcia-Garibay, G.C. Bazan
Phys. Chem. Chem. Phys., 16 (2014) 12980.
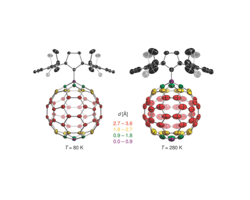
Thermal displacement ellipsoids at 80 and 280 K indicate the presence of a rapid rotation of the C60 fragment around the C-C bond joining it to the carbene at high temperature. The color code indicates the distance of the atoms on the fullerene to the rotation axis.
A new fullerene structure was recently obtained from the reaction of a N-heterocyclic carbene and C60. The molecular features of the zwitterionic adduct can be described as a molecular rotor with the fullerene cage acting as the rotator that spins about one distinct axis given by its C–C single bond linkage with the imidazolium heterocycle stator. Variable temperature single-crystal x-ray diffraction experiments (80 K < T < 480 K) carried out to investigate the rotational dynamics of the fullerene group revealed atomic displacement parameters consistent with fast rotation of the highly symmetric fullerene in the solid state, whereas the imidazolium unit remains in a fixed position and therefore represents the stator. DFT and semiempirical calculations were applied to get insight into the profile of the rotational potential of the fullerene unit, particularly considering interactions with the neighboring molecules in the crystal lattice. The results indicate that the crystal environment leads to the presence of one lowest energy minimum that is connected to seven others that are slightly higher in energy through rotational barriers of approximately 1.5–2.5 kcal mol-1.
