Unexpected Reactivity of Amidogen Radical in the Gas Phase Degradation of Nitric Acid
Unexpected Reactivity of Amidogen Radical in the Gas Phase Degradation of Nitric Acid
J.M. Anglada, S. Olivella, A. Solé
J. Am. Chem. Soc., 136 (2014) 6834.
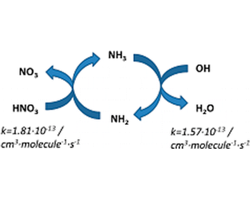
Catalytic cycle involving the oxidation of NH3 by OH radical and the reaction between amidogen radical and nitric acid
The gas phase reaction between nitric acid and amidogen radical has been investigated employing high level quantum-mechanical electronic structure methods and variational transition state theory kinetic calculations. Our results show that the reaction proceeds through a proton coupled electron transfer mechanism with a rate constant of 1.81 × 10–13 cm3·molecule–1·s–1 at 298 K. This value is similar to the rate constants for the reactions of hydroxyl radical with either ammonia or nitric acid. An analysis of these data in the context of the chemistry of the atmosphere suggests that the amidogen radical, formed in the oxidation of ammonia by hydroxyl radical, reacts with nitric acid regenerating ammonia. On the basis of these findings, we propose a potential new catalytic-like cycle which couples the oxidation of ammonia by hydroxyl radical and the reaction of nitric acid with amidogen radical in the Earth’s atmosphere.
