From widely accepted concepts in coordination chemistry to inverted ligand fields
From widely accepted concepts in coordination chemistry to inverted ligand fields
R. Hoffmann, S. Alvarez, C. Mealli, A. Falceto, T. J. Cahill, T. Zeng, G. Manca.
Chem. Rev., 116 (2016) 8173.
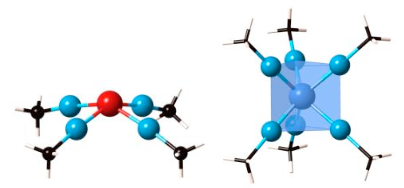
Proposed complexes with inverted ligand fields: [Rh(AlMe)4]+ i [Mo(AlMe)6].
We have designed a strategy for inverting the normal splitting of the metal d orbitals in the presence of a ligand field and computationally constructed several examples with a hypothetical but not unrealistic AlCH3 ligand. Special attention is paid to the square-planar case, exemplified by [Cu(CF3)4]-, in which Snyder had the foresight to see a case of an inverted field, with the empty valence orbital being primarily ligand centered, the dx2-y2 orbital heavily occupied, in what would normally be called a Cu(III) complex. For [Cu(CF3)4]- we provide theoretical evidence from electron distributions, geometry of the ligands, thermochemistry of molecule formation, and the energetics of abstraction of a CF3 ligand by a base, all consistent with oxidation of the ligands in this molecule. Exploration of inverted ligand fields helps us see the continuous, borderless transition from transition metal to main group bonding. We also give voice to a friendly disagreement on oxidation states in these remarkable molecules.
