Computational Sudy of the Aza-Michael Addition of the Flavonoid (+)-Taxifolin in Inhibition of β-Amyloid Fibril Aggregation
T. Ginex, M. Trius, F. J. Luque.
Chem. Eur. J., 24 (2018) 5813-5824.
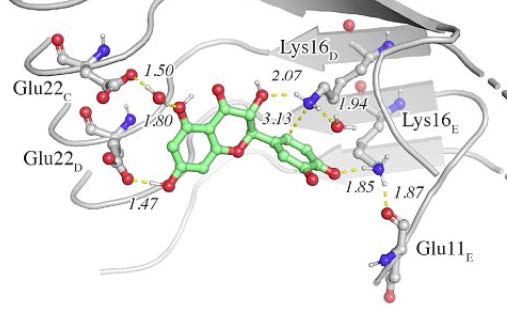
Detail of the binding of (+)-taxifolin to β-amyloid.
The formation of covalent adducts with amyloid fibrils that interfere with the aggregation process opens novel avenues for intervention in amyloidogenic diseases. This study examines the mechanism of formation of a covalent adduct between the oxidized form of (+)-taxifolin and β- amyloid (Aβ42). The results support the involvement of a specific recognition motif that enables the chemical reaction with Aβ42. Thus, (+)-taxifolin binds to the hydrophobic groove delimited by the edges defined by Lys16 and Glu22 residues in the fibril. This mechanism, which may explain the enhanced anti-aggregating activity of oxidized flavonoids, holds promise for developing disease-modifying therapies.
