Nido-Cage···pi bond: a non-covalent interaction between boron clusters and aromatic rings and its applications.
H. Yan, D. Tu, J. Poater, M. Solà.
Angew. Chem. Int. Ed., 59 (2020) 9018-9025.
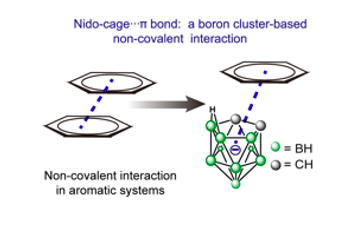
Non‐covalent interactions involving multicenter multielectron skeletons such as boron clusters are rare. Now, a non‐covalent interaction, the nido‐cage⋅⋅⋅π bond, is discovered based on the boron cluster C2B9H12− and an aromatic π system. The X‐ray diffraction studies indicate that the nido‐cage⋅⋅⋅π bonding presents parallel‐displaced or T‐shaped geometries. The contacting distance between cage and π ring varies with the type and the substituent of the aromatic ring. Theoretical calculations reveal that this nido‐cage⋅⋅⋅π bond shares a similar nature to the conventional anion⋅⋅⋅π or π⋅⋅⋅π bonds found in classical aromatic ring systems. This nido‐cage⋅⋅⋅π interaction induces variable photophysical properties such as aggregation‐induced emission and aggregation‐caused quenching in one molecule. This work offers an overall understanding towards the boron cluster‐based non‐covalent bond and opens a door to investigate its properties.
