A Model Potential for Acetonitrile: from Small Clusters to Liquid
A Model Potential for Acetonitrile: from Small Clusters to Liquid
M. Albertí, A. Amat, F. De Angelis, F. Pirani
J. Phys. Chem. B., 117 (2013) 7065.
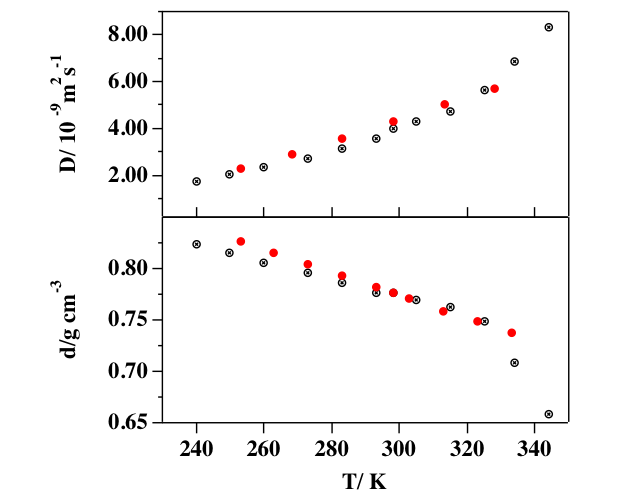
The CH3CN-CH3CN and ion-CH3CN (ion= Li+, Na+, K+, I– ) interaction potentials, allowing the modelitation of both pure acetonitrile and its solutions, are presented. The interaction potentials have been constructed to be used in the study of dye-sensitized solar cells. The first step in the construction of the potential model is to assume the total separability of electrostatic and nonelectrostatic interactions, the late being represented by menas of the Improved Lennard Jones function, whose relevant parameters have been derived from values of the polarizability assigned to the ions and to different groups on the CH3CN molecule, compatible with the whole molecular polarizability. By means of Molecular Dynamics simulations and the application of the interaction potential, small clusters of acetonitrile, (CH3CN)2,3,4 , ion-acetonitrile clusters of the ion- (CH3CN)36 type and liquid acetonitrile have been investigated.
