Keys for the Existence of Stable Dimers of Bis-tetrathiafulvalene (bis-TTF)-Functionalized Molecular Clips Presenting [TTF]·+···[TTF]·+ Long, Multicenter Bonds at Room Temperature
Keys for the Existence of Stable Dimers of Bis-tetrathiafulvalene (bis-TTF)-Functionalized Molecular Clips Presenting [TTF]·+···[TTF]·+ Long, Multicenter Bonds at Room Temperature
M. Fumanal, M. Capdevila-Cortada, J. S. Miller, J. J. Novoa.
J. Am. Chem. Soc. 135 (2013) 13814.
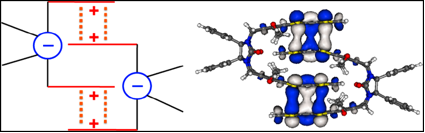
A computacional study has been performed to determine the keys governing the existence, in acetonitrile solutions, of dimers of (bis-TTF)-diphenylglycoluril molecular clips (clip2n+) that are stable at room temperature for n≤4. Electronic absorption studies suggested that they present [TTF]l+···[TTF]m+ interactions at r.t.. We show that all clip2n+ n≤4 present an optimum geometry that has three short [TTF]l+···[TTF]m+ interactions, and the computed ΔG(298K) matches the experimental data on the stability of these dimers. Our study traces the origin of their stability to (1) the zwitterionic character of their charge distribution, (2) the geometrical shape of the dimer, that allows the formation of two short contacts involving the + charged TTF group and the – charged central ring, (3) the presence of three short contacts among the TTF groups, which become two long bonds and one vdW interaction when the four TTF groups host a 1+ charge, and (4) the net stabilizing effect of the solvent.
