Experimental guided ion beam and ab initio studies of the reactive processes in gas phase i-C3H7Br and i-C3H7OH collisions with potassium ions
Experimental guided ion beam and ab initio studies of the reactive processes in gas phase i-C3H7Br and i-C3H7OH collisions with potassium ions
E. López, J.M. Lucas, J. de Andrés, M. Albertí, J.M. Bofill, D. Bassiand A. Aguilar.
J. Chem. Phys., 141 (2014) 164310.
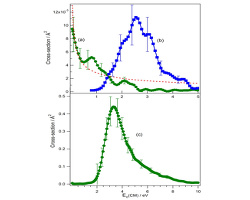
Cross-section CM energy dependences in K+ + i-C3H7Br collisions for: (a) [K-i-C3H7Br]+ adduct formation (●); (b) dehydrohalogenation reaction (■); (c) adduct decomposition reaction. Discontinuous line: LGS model (energies are given in the CM frame)
Collisions between K+ and i-C3H7Br and i-C3H7OH molecules, all in their electronic ground state, have been studied in our laboratory in the 0.10-10.00 eV center of mass (CM) collision energy range, using the radiofrequency-guided ion beam (RF-GIB) technique. In K+ + i-C3H7Br collisions KHBr+ formation is observed and quantified, while the analogous KH2O+ formation by i-C3H7OH dehydration was hardly detected. Moreover, formation of the [K–i-C3H7Br]+ and [K–i-C3H7OH]+ adducts and their decomposition leading to C3H7+ and KBr or KOH, respectively, have been observed. Absolute reaction cross-sections are measured as a function of the CM energy and the thermal rate constant at 303 K has been calculated for KHBr+ formation. Ab initio structure calculations at the MP2 level gave information about the relevant features of the potential energy surfaces where reactions take place adiabatically for both systems and allowed a qualitative interpretation of the experimental data to be proposed.
