Theoretical study of the free energy surface and kinetics of the hepatitis C virus NS3/NS4A serine protease reaction with the NS5A/5B substrate. Does the generally accepted tetrahedral intermediate really exist?
Theoretical study of the free energy surface and kinetics of the hepatitis C virus NS3/NS4A serine protease reaction with the NS5A/5B substrate. Does the generally accepted tetrahedral intermediate really exist?
J. A. Martínez-González, M. González, L. Masgrau, R. Martínez
ACS Catal. in press (dx.doi.org/10.1021/cs5011162).
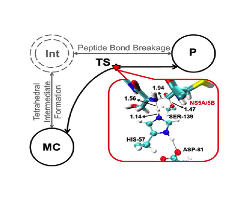
Concerted acylation mechanism found for the reaction of the NS3/NS4A serine protease and the NS5A/5B substrate, compared with the generally proposed two-step mechanism. A molecular representation of the transition state characterized is also shown.
The SCC-DFTB/MM and EA-VTST/MT theoretical methods were used to analyze the mechanism and calculate the rate constant of the NS3/NS4A protease + NS5A/5B acylation reaction, which is very important in the vital cycle of the hepatitis C virus. A concerted reaction mechanism with a single transition state (TS) has been determined, in contrast with the proposed general two-steps serine protease acylation mechanism. This is related to the fact that the enzyme is particularly efficient for NS5A/5B. The acylation TS found here can be a good initial structure in the search of NS3/NS4A inhibitors based on TS analogs. Moreover, the calculated and experimental phenomenological free energy barriers only differ by 2.3 kcal mol-1 (although this leads to a significant discrepancy between calculated and experimental rate constants), and the rest of calculated kinetic parameters (kinetic isotopic effect (H/D), tunneling, and recrossing) agree with the expected behaviour for the studied reaction.
