Absence of a Stable Secondary Structure Is Not a Limitation for Photoswitchable Inhibitors of βArrestin/ βAdaptin 2 ProteinProtein Interaction
Absence of a Stable Secondary Structure Is Not a Limitation for Photoswitchable Inhibitors of βArrestin/ βAdaptin 2 ProteinProtein Interaction
A. Martín-Quirós, L. Nevola, K. Eckelt, S. Madurga, P. Gorostiza, E. Giralt.
Chemistry & Biology, 22 (2015) 31.
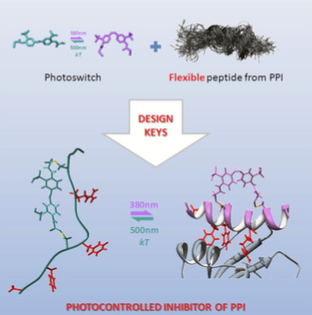
Design of a flexible photoswitchable inhibitor.
Many protein-protein interactions (PPIs) are mediated by short, often helical, linear peptides. Molecules mimicking these peptides have been used to inhibit their PPIs. Recently, photoswitchable peptides with little secondary structure have been developed as modulators of clathrin-mediated endocytosis. Here we perform a systematic analysis of a series of azobenzene- crosslinked peptides based to assess the relevance of secondary structure in their interaction with β-adaptin 2 and to identify photoswitchable inhibitors. We observe that flexible structures show a greater inhibitory capacity and enhanced photoswitching ability and that the absence of helical structures in free inhibitor peptide is not a limitation for their capacity. Therefore, our designed inhibitors expand the field of potential inhibitors of PPIs to the wide group of flexible peptides, and we argue against using a stable secondary structure as a sole criterion when designing PIPPI candidates.
