A Front-Face ‘SNi synthase’ engineered from a retaining ‘double-SN2’ hydrolase
A Front-Face ‘SNi synthase’ engineered from a retaining ‘double-SN2’ hydrolase
J. Iglesias-Fernández, S. M. Hancock, S. S. Lee, M. Khan, J. Kirkpatrick, N. J. Oldham, K. McAuley, A. Fordham-Skelton, C. Rovira, B. G. Davis.
Nat. Chem. Biol., 13 (2017) 874.
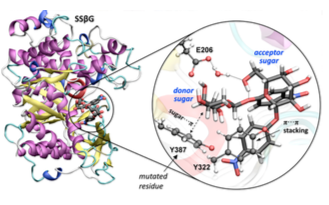
Close view of the active site of the modified enzyme (Sulfolobus solfataricus β-glycosidase, SSβG) showing the two
bound carbohydrate substrates ready for glycosidic bond synthesis.
Sugar or carbohydrate synthesis is important for the development of diagnostic tests, vaccines and new drugs. Conventional chemical synthesis in the laboratory is usually expensive and cumbersome; therefore great efforts are being made to use enzymes, which are natural catalysts. In this work we modified a robust glycosidase enzyme, which normally degrades carbohydrates, to do the opposite and synthetize carbohydrates. The modification consists on a single mutation of one active site amino acid. The main novelty of the work is that the engineered enzyme operates via a reaction mechanism (named front-face or SNi-like) that had not been previously observed in glycosidases, opening new possibilities for carbohydrate synthesis. Molecular biology techniques, together with the modeling of the reaction mechanism using a combination of molecular dynamics and quantum mechanics methods, have unraveled the new enzyme catalytic mechanism.
