Ionization and Conformational Equilibria of Citric Acid: Delocalized Proton Binding in Solution
Ionization and Conformational Equilibria of Citric Acid: Delocalized Proton Binding in Solution
S. Madurga, M. Nedyalkova, F. Mas, J. L. Garcés.
J. Phys. Chem. A, 121 (2017) 5894.
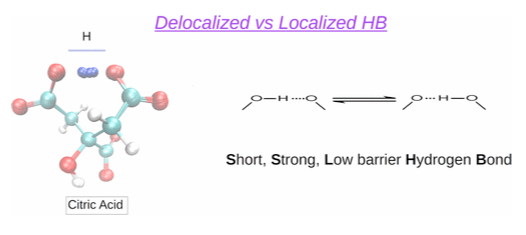
Calculation of localized and delocalized hydrogen bonds in the citric acid molecule.
The microspeciation of citric acid is studied by analyzing NMR titration data using ab initio and statistical mechanics techniques (site binding models). With this aim, ab initio MP2 calculations using the SMD polarizable continuum model for the solvent were performed and the fully roto-microspeciation of citric acid was elucidated. The results reveal that the exchange of the proton through the hydrogen bonds is in some cases produced without energetic barrier. This effect is specially relevant in the di-ionized form, with all the most stable conformations forming a Short, Strong, Low barrier (SSLB) hydrogen bond, which together would constitute the only microstate detected by NMR.
