Origin of bistability in the butyl-substituted spirobiphenalenyl-based neutral radical material
Origin of bistability in the butyl-substituted spirobiphenalenyl-based neutral radical material
M. Fumanal, J. J. Novoa, J. Ribas-Ariño.
Chem. Eur. J., 23 (2017) 7772.
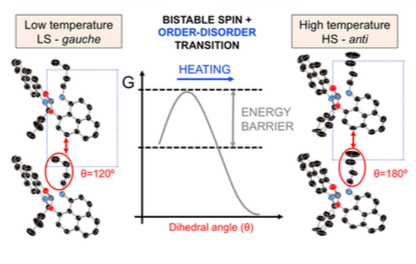
One of the most remarkable bistable materials reported so far is made of π dimers of a butyl-substituted spirobiphenalenyl boron radical (butyl-SBP). The phase transition of this material, which is accompanied by changes in its optical, conductive, and magnetic properties, occurs with a hysteretic loop 25 K wide centered at 335 K. In this computational study, we deciphered the origin of this hysteresis. The phase transition of butyl-SBP consists of a spin transition of the constituent π dimers coupled with an order–disorder transition involving the butyl chains linked to phenalenyl rings. Below 335 K, the butyl chains adopt a gauche conformation, whereas above 335 K, the butyl chains are in an anti conformation and exhibit dynamic disorder. The gauche -> anti conformational rearrangement triggers the spin transition of the π dimers and is responsible for the hysteretic behavior of butyl-SBP. Our results show that coupling a spin switch with a conformational switch provides a promising strategy in the design of new bistable materials.
