The photophysics of naphthalene dimers controlled by sulfur bridge oxidation
The photophysics of naphthalene dimers controlled by sulfur bridge oxidation
C. Climent, M. Barbatti, M. O. Wolf, C. J. Bardeen, D. Casanova.
Chem. Sci. 8 (2017) 4941-4950.
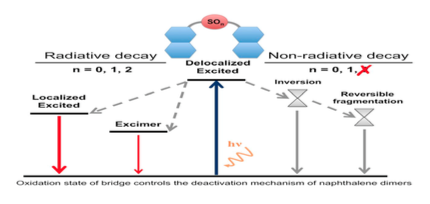
Schematic Jablonski diagram of covalent naphthalene dimers according to the oxidation state of the bridging sulfur (S, SO, SO2).
In this work we investigate the photophysics of naphthalene dimers covalently linked by a sulfur atom. We explore and rationalize how the oxidation state of the sulfur-bridging atom directly influences the photoluminescence of the dimer by enhancing or depriving its radiative and nonradiative relaxation pathways. In particular, we discuss how oxidation controls the amount of electronic transfer between the naphthalene moieties and the participation of the SOn bridge in the low-lying electronic transitions. We identify the sulfur electron lone-pairs as crucial actors in the non-radiative decay of the excited sulfide and sulfoxide dimers, which are predicted to proceed via a conical intersection (CI). Concretely, two types of CI have been identified for these dimers, which are associated with the photo-induced pyramidal inversion and reverse fragmentation mechanisms found in aryl sulfoxide dimers. The obtained results and conclusions are general enough to be extrapolated to other sulfur-bridged conjugated dimers, therefore proportionating novel strategies in the design of strongly photoluminescent organic molecules with controlled charge transfer.
