Design of an interface peptide as new inhibitor of humanglucose-6-phosphate dehydrogenase
Design of an interface peptide as new inhibitor of humanglucose-6-phosphate dehydrogenase
C. Obiol-Pardo, G. Alcarraz-Vizánb, S. Díaz-Moralli, M. Cascante, J. Rubio-Martinez
Journal of Molecular Graphics and Modelling, 49 (2014) 110
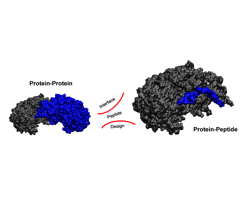
Design of interface peptides
Glucose-6-phosphate dehydrogenase (G6PDH) is an essential enzyme involved in the first reaction of the oxidative branch of the pentose phosphate pathway (PPP). Recently, G6PDH was suggested as a novel target protein for cancer therapy as one of the final products of the PPP, ribose-5-phosphate, is necessary for nucleic acid synthesis and tumor progression. After analyzing the protein–protein interface of the crystal structure of human G6PDH by means of molecular dynamics simulations, we designed six interface peptides based on the natural sequence of the protein. The three most promising peptides, as predicted by binding free energy calculations, were synthesized and one of them was confirmed as a novel inhibitor of human G6PDH in experimental assays. Together, the active peptide found and its suggested binding mode proposes a new strategy for inhibiting this enzyme and should aid the further design of novel, potent and non-peptidic G6PDH inhibitors.
