Effects of Dimethyl Sulfoxide on Lipid Membrane Electroporation
Effects of Dimethyl Sulfoxide on Lipid Membrane Electroporation
M.L. Fernández, R. Reigada
J. Phys. Chem. B, 118 (2014) 9306.
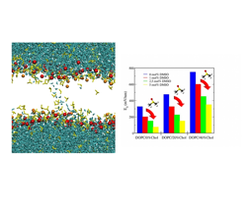
DMSO (yellow) facilitates the formation of electropores in the membrane and the passage of water molecules (cyan). This effects causes a reduction of the minimum electroporation field.
Pores can be generated in lipid membranes by the application of an external electric field or by the addition of particular chemicals such as dimethyl sulfoxide (DMSO). Molecular dynamics (MD) has been shown to be a useful tool for unveiling many aspects of pore formation in lipid membranes in both situations. By means of MD simulations, we have addressed the formation of electropores in cholesterol-containing lipid bilayers under the influence of DMSO. We show how a combination of physical and chemical mechanisms leads to more favorable conditions for generating membrane pores and, in particular, how the addition of DMSO to the medium significantly reduces the minimum electric field required to electroporate a lipid membrane. The strong alteration of membrane transversal properties and the energetic stabilization of the hydrophobic pore stage by DMSO provide the physicochemical mechanisms that explain this effect.
