A molecular dynamics study of the evolution from the formation of the C6F6–(H2O)n small aggregates to the C6F6 solvation
A molecular dynamics study of the evolution from the formation of the C6F6–(H2O)n small aggregates to the C6F6 solvation
M. Albertí, A. Amat, A. Aguilar, F. Huarte-Larrañaga, J.M. Lucas, F. Pirani and A. Aguilar.
Theor. Chem. Acc., 134 (2015) 61.
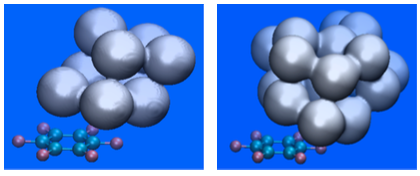
Isosurface (isovalue 0.5) plot of the H2O probability density in the C6F6-(H2O)9 (left-hand side) and the C6F6-(H2O)18 (right-hand side) aggregates. Results correspond to Molecular Dynamics simulations at a temperature of 20 K
In this study, the evolution from the formation of small clusters to the solvation of hexafluorobenzene is investigated by means of Molecular Dynamics simulations. For this purpose, the non permanent charge interaction contributions are described using an Improved Lennard-Jones model (ILJ) in combination with the electrostatic energy calculated in agreement with the permanent electric quadrupole and dipole moments of C6F6 and H2O, respectively. To test the potential energy function, three different approaches of H2O-C6F6 have been considered and BSSE-corrected energies at the CCSD(T)/aug-cc-pVTZ level have been calculated for the three approaches. By using the constructed force field, the structure and energetics of some small aggregates, C6F6-(H2O)n (n = 1–6 ), the formation of the first solvation shell C6F6-(H2O)n (n = 9–36) and the solvation of C6F6 by 400 molecules of H2O have been investigated. The small aggregates and the formation of the first solvation shell have been simulated using a microcanonical (NVE) ensemble of particles, while an isobaric–isothermal ensemble (NpT) has been used to investigate the solvation of of C6F6. In order to approximate the system formed by one C6F6 molecule and 400 H2O molecules to a large (infinite) system, periodic boundary conditions have been imposed in the simulation of the solvation C6F6. It has been found that the first solvation shell is only closed when the number of water molecules exceeds those needed to complete it. This fact evidences a high competition between C6F6-H2O and H2O-H2O interactions.
