Diffusion of H2 and D2 confined in single-walled carbon nanotubes: quantum dynamics and confinement effects
Diffusion of H2 and D2 confined in single-walled carbon nanotubes: quantum dynamics and confinement effects
M. Mondelo-Martell, F. Huarte-Larrañaga
J. Phys. Chem. A 120 (2016) 6501
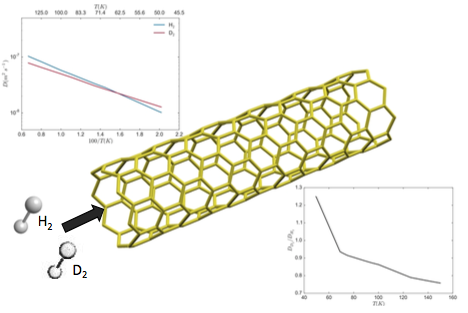
Center figure is an illustration of the diffusion process of H2 and D2 in a SWCNT. Top figure plots de computed diffusion constants as a function of 100/T. The bottom figure represents the selectivity coefficient as a function of temperature.
In this publication we present quantum dynamics calculations of the diffusion constant of H2 and D2 along a single-walled carbon nanotube at temperatures between 50 and 150 K. We calculate the respective diffusion rates in the low pressure limit by adapting the flux correlation function formalism from the chemical dynamics field to the hopping model used in diffusion simulations. Two different Potential Energy Surfaces are employed to model the C-H interaction. Our results predict a usual kinetic isotope effect, with H2 diffusing faster than D2 in the higher temperature range, but a reverse trend at temperatures below 50-70 K. These findings are consistent with experimental observation in similar systems, and can be explained by the different effective size of both isotopes resulting from their different Zero Point Energy.
