Computational Catalysis for A Greener Future
By WIREs Comput Mol Sci. (2021) e1530 Authors
Computational techniques are increasingly helping researchers to speed up catalysts tuning and design
Catalysis is no doubt a key technology in the overall chemical industry contributing to many aspects of our modern society including fuels and many commodities. Catalysis is without any doubt a cornerstone to address the current environmental challenges in energy and food production, gas emissions with concomitant consequences for health. However, many of the present challenges cannot be satisfactorily addressed with the present catalysts and, therefore, scientists are working on the development of new or improved catalysts to foster their activity, selectivity, and the operando conditions. The roadmap towards this objective is complex and requires going beyond expensive and inefficient trial and error approaches. The recent advances in computational materials science together with the ever-growing computational capability with easy access to modern supercomputers with thousands of cores and the appropriate parallel codes make it possible an efficient and iterative interplay between experimental and computational research that is more and more playing a role in catalyst discovery and engineering.
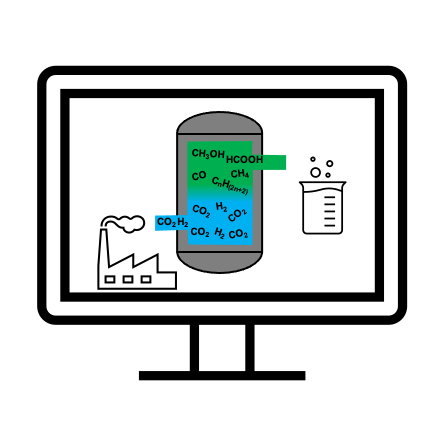
In a recent review published in WIREs Computational Molecular Science, Dr. Morales-García and Profs. Viñes, Gomes and Illas introduce the main concepts and methods of modern computational catalysis and illustrate them by analyzing the properties of a series of catalytic materials that are being investigated to contribute to solve the problem of excessive concentration of the CO2 greenhouse gas in the Earth atmosphere by catalytic conversion to useful chemicals such as CO, a syngas component, or methanol that can be used as a fuel thus contributing to a circular CO2 economy.
“The catalytic conversion of CO2 is one of the main challenges of our society and combining experiments and computational modeling is fundamental to succeed before it is too late” point out the authors, “carbon monoxide (CO), formic acid (HCOOH), methane (CH4) and ethylene (C2H4) are some of the fuels and fuel precursors obtained from CO2 catalytic conversion processes”. From a theoretical and computational viewpoint, the challenge is to shed light on the aspects where experiments alone are not sufficient, this involves the nature of active sites, the understanding of the underlying chemical mechanism and the time evolution of catalyst structure with the environment under the operando conditions at which the chemical reaction takes place. Here, multiscale modeling emerges as a powerful tool increasingly used in chemical engineering as well.
For more information check out the original publication:
