Van der Waals crust behind simple parameter that can describe chemical bonds
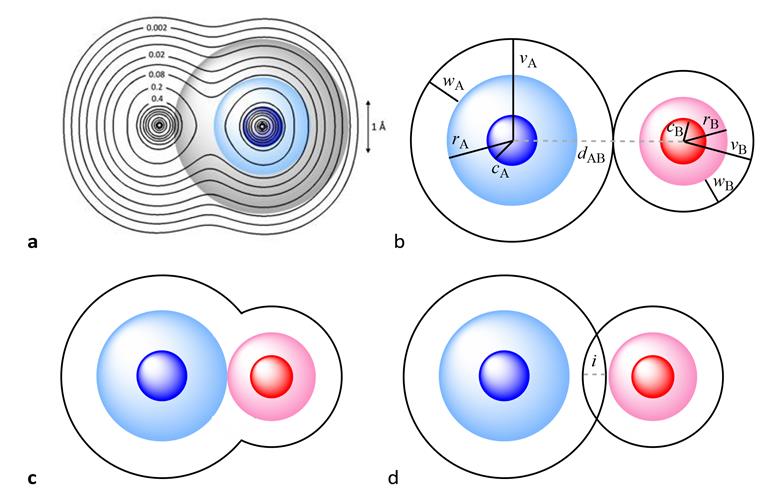
‘The [van der Waals] crust is at a distance from the nucleus larger than the covalent radius but shorter than the van der Waals radius, it’s a region where a host of weakly attractive interactions occur,’ explains Santiago Álvarez, from the University of Barcelona, who developed the parameter alongside Jorge Echevarría from the University of Zaragoza.
They have devised a new parameter for comparing and understanding bonds. It describes the degree of interpenetration of the van der Waals crusts of two atoms, which they call the penetration index. It can replace previous distance measurements with simple calculations about crust overlap, and be applied to bonds ranging from strongly covalent multiple bonds to weak London dispersion forces.
The duo rationalised that a penetration index based on the overlap between the van der Waals crusts of two atoms can describe all chemical bonds between atoms. A single covalent bond would have a value of 100% on the penetration index, with total overlap of the van der Waals crust. A traditional van der Waals force would have a value of 0%. There are instances of values outside this range, with double and triple bonds having values higher than 100%. Values in the negative range indicate interactions that occur outside of the van der Waals crust.