Disruption of the Chemical Environment and Electronic Structure in p-Type Cu2O Films by Alkaline Doping.
Disruption of the Chemical Environment and Electronic Structure in p-Type Cu2O Films by Alkaline Doping
F. Caballero-Briones, A. Palacios-Padros, O. Calzadilla, I. de P. R. Moreira, F. Sanz,
J. Phys. Chem. C 116 (2012) 13524

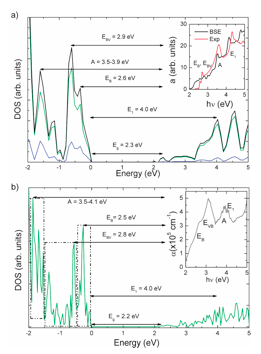
Left: topographic AFM images (2 × 2 mm2) showing the surface morphology of Cu2O films doped with (a) Li, (b) Na, (c) K and (d) Cs. Notice the reduction in grain size from Li to Cs doped materials. Right: Calculated electronic density of states for (a) Cu2O and (b) Cu2O:K. A rigid band model has been used to interpret the nature of the states defining Eg as well as the EB, EBV, A, and E1 peaks observed in the corresponding absorption spectra.
This work is a collaboration with the Bioelectrochemistry and Nanotechnology group leaded by Prof. F. Sanz. Here we describe and analyze the interesting properties of Cu2O films doped with alkaline ions (Li+, Na+, K+ and Cs+) prepared by Cu anodization. The main effects of the alkaline doping on the optical properties were a reduction in the direct band gap and an approach of the acceptor level edge to the maximum of the valence band, which induce important changes in the observed optical response. First principles band structure calculations on models for the alkaline doped Cu2O systems suggest that the main effect of the alkaline substitution of copper atoms consists in polarizing the O states, which causes a reduction in the insulating gap and splitting of the density of states just below the Fermi level. The nature of the oxygen-dopant interaction was attractive for Li, slightly repulsive for Na and clearly repulsive for K and Cs. This fact provides an explanation for the observed surface accumulation of K+ and Cs+ that hinders vacancy diffusion and blocks film growth, leading to a reduction of roughness and thickness of crystallites as the ion size increases.
