Molecular Determinants of Bim(BH3) Peptide Binding to Pro-Survival Proteins.
Molecular Determinants of Bim(BH3) Peptide Binding to Pro-Survival Proteins
L. Delgado-Soler, M. Pinto, K. Tanaka-Gil , J. Rubio-Martinez.
J. Chem. Inf. Model., 52 (2012) 2107.
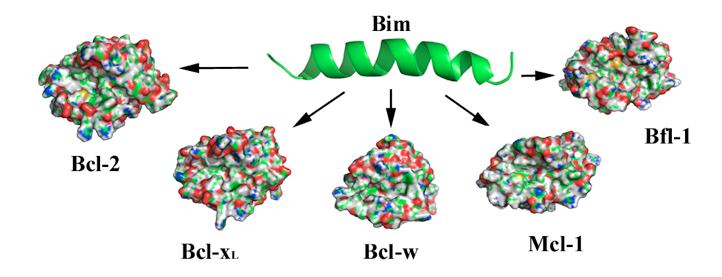
The Bcl-2 family proteins are well-characterized regulators of the intrinsic apoptotic pathway. Proteins within this family can be classified as either prosurvival or prodeath members. The balance between them present at the mitochondrial membrane is what determines if the cell lives or dies. For this reason, the design of small molecules inhibiting the proapoptotic proteins has emerged as a promising therapeutic strategy for cancer treatment during the last years. Although the precise mechanism by which this family of proteins regulates apoptosis is still poorly understood, it is well accepted that anti- and proapoptotic proteins modulate their opposite functions through heterodimerization. The experimental structures of antiapoptotic proteins complexed with peptides derived from the BH3 domain of proapoptotic proteins has provided the first insights into the molecular mechanism of the heterodimerization of this family of proteins. In these complexes, the antiapoptotic protein forms a hydrophobic cleft on its surface wherein the hydrophobic face of an amphipathic α-helix containing the BH3 domain of the proapoptotic protein binds. In this scenario, it was postulated that small molecules mimicking the BH3 α-helix of proapoptotic proteins may be able to bind to the same hydrophobic groove of antiapoptotic Bcl-2 family members blocking their heterodimerization and leading to apoptosis. One of the most important considerations to bear in mind when designing BH3 mimetics is the different affinity of BH3 domains for individual antiapoptotic proteins. Bim and Puma are the only proapoptotic proteins that bind to all pro-survival proteins with similar affinities. Thus, mimetics of any of these proteins should be able to bind to all the antiapoptotic proteins and should represent a first step in the development of new anticancer agent.
