The Large Scale Conformational Change of the Human DPP III−Substrate Prefers the “Closed” Form.
The Large Scale Conformational Change of the Human DPP III−Substrate Prefers the “Closed” Form
A. Tomic, M. González, S. Tomic.
J. Chem. Inf. Model., 52 (2012) 1583.
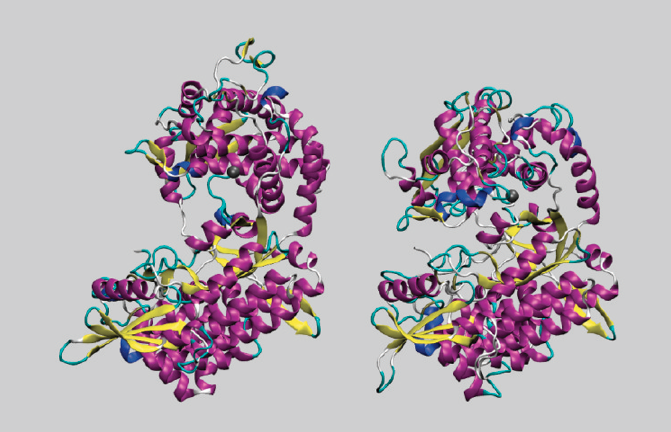
Initial structure of DPP III (left) and structure obtained after 101 ns of MD simulation (right).
The wide space available between the two domains and the broad substrate specificity that presents the human variant of the dipeptidil peptidase III (DPP III) enzyme suggests that it may experience significant conformational changes. And indeed this is what has been observed in Molecular Dynamics (MD) simulations (> 100 ns), and free energy calculations show that the preferred synthetic substrate (Arg-Arg-2-naphtylamide) has a stronger binding to the enzyme in the “closed” conformation than in the “open” one. Our assumption that the Asp372 residue plays a crucial role in the interdomain closing was evidenced by MD simulations on the Asp372Ala variant. According to the MM-PBSA calculations, the electrostatic component of the solvation free energy is found to be larger for the protein in its closed form than for the less compact form. However, the gain in entropy caused by the water molecules released from the interdomain space balances this negative effect. So, we have a very interesting process which is driven by the increase of entropy, in accordance with what has been suggested very recently by the experiments.
