Comparing the catalytic activity of the water gas shift reaction on Cu(321) and Cu(111) surfaces: step sites do not always enhance the overall reactivity
Comparing the catalytic activity of the water gas shift reaction on Cu(321) and Cu(111) surfaces: step sites do not always enhance the overall reactivity
H. Prats, P. Gamallo, F. Illas, R. Sayós.
J. Cat., 342 (2016) 75.
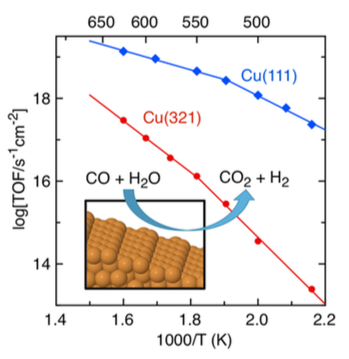
A Density Functional Theory based first-principles kinetic Monte Carlo (kMC) study of the water gas shift reaction on the stepped Cu(321) surface is presented. We use the recently developed graph-theoretical kMC approach coupled with cluster expansion Hamiltonians to model the coverage-dependent energy barriers for the different surface processes, including adsorption/desorption, diffusion and other elementary chemical reactions, totalling 36 elementary steps, which allow two possible competitive mechanisms: surface redox and associative COOH. All results are compared to a previous kMC study on Cu(111). Both mechanisms are observed for Cu(321) surface with different extension, whereas the associative COOH one was the dominant for Cu(111). The present study shows that, in spite of encompassing lower activation energy barriers, stepped surfaces do not necessarily have an overall larger catalytic activity. Coverage effects and the significant contribution of some of the reverse processes are behind this behaviour.
