Dehydrohalogenation and dehydration reactions of i-C3H7Br and i-C3H7OH by sodium Ions studied by guided ion beams techniques and quantum chemical methods
Dehydrohalogenation and dehydration reactions of i-C3H7Br and i-C3H7OH by sodium Ions studied by guided ion beams techniques and quantum chemical methods
E. López, J. M. Lucas, J. De Andrés, M. Albertí, J. M. Bofill, A. Aguilar.
J. Phys. Chem. A, 120 (2016) 4758.
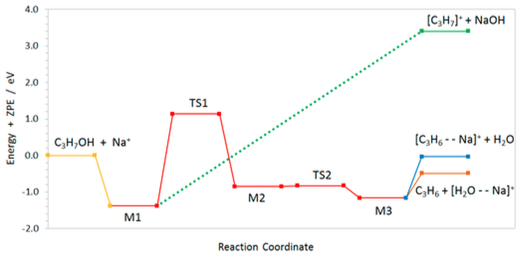
Schematic ZPE profile along the reaction path calculated at the ab initio MP2 level on the singlet ground state of the Na+ + i-C3H7OH collision system showing the different minima (M) and transition states (TS1) on the PES and their IRC connectivity.
Elimination reactions of gas phase i-C3H7Br and i-C3H7OH induced by collision with Na+ in their electronic ground state were studied experimentally in a radiofrequency-guided ion beam (RF-GIB) apparatus and covering the 0.10 – 10.00 eV center of mass energy range. Na+ + i- C3H7Br leads to form [Na–i-C3H7Br]+, [C3H6–Na]+, [HBr–Na]+ and C3H7+ while for Na+ + i-C3H7OH only [Na–i-C3H7OH]+, [H2O–Na]+ and C3H7+ were found. For all products absolute excitation functions were measured and from them rate constants for the formation of [C3H6–Na]+, [HBr– Na]+ and [H2O–Na]+ at 303 K were obtained. Ab initio structure calculations at the MP2 level gave information on potential energy surfaces/PES) where elimination and decomposition reactions evolve adiabatically, characterizing different minima and transition state connected along the IRC. PES topology features allow a qualitative interpretation of the experimental data and show the role of the sodium ion as a catalyst in elimination reactions.
