The Atmospheric Oxidation of HONO by OH, Cl, and ClO Radicals
The Atmospheric Oxidation of HONO by OH, Cl, and ClO Radicals
J. M. Anglada, A. Solé.
J. Phys. Chem. A 121 (2017) 9698.
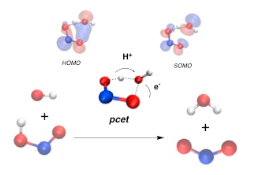
The atmospheric oxidation of nitrous acid by hydroxyl radical, chlorine atom and chlorine monoxide radical was investigated with high-level theoretical methods. Nitrous acid has two conformers (cis and trans) and we found a reaction path for the oxidation of each of these conformers with the radicals considered. In all cases, the oxidation of the cis conformer is much more favorable than the oxidation of the trans conformer. Interestingly all transition states in these oxidation processes follow a proton coupled electron transfer mechanism. Our computed rate constant at 298 K for the reaction of cis-HONO + •OH is 4.83 x 10-12 cm3 molecule-1 s-1, in excellent agreement with their experimental values (4.85 x 10-12 and 6.48 x 10-12 cm3 molecule-1s-1). For the trans-HONO + •OH reaction our calculated rate constant at 298 K is 9.05 x 10-18cm3 molecule-1 s-1, and we computed an effective rate constant for the oxidation of the whole nitrous acid by hydroxyl radical of 1.81 x 10-12 cm3 molecule-1 s-1. For the oxidation of nitrous acid by chlorine atom we predict greater rate constants (7.38 x 10-11, 3.33 x 10-15, and 2.76 x 10-11 cm3 molecule-1 s-1, for the cis and trans conformers and for the whole HONO), these results suggesting that this reaction should contribute to the tropospheric oxidation of nitrous acid, especially in marine boundary areas, and to the formation of tropospheric ozone. For the oxidation of nitrous acid by chlorine monoxide we predict rate constants roughly 6 orders of magnitude smaller than the oxidation by chlorine atom, and therefore we consider that this process should play a minor role in the troposphere.
