Double CH activation of a masked cationic bismuth amide
B. Ritschel, J. Poater, H. Dengel, F. M. Bickelhaupt, C. Lichtenberg.
Angew. Chem. Int. Ed., 57 (2018) 3825-3829.
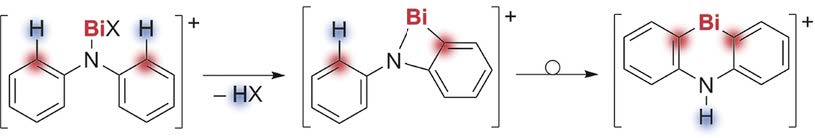
The transformation of CH bonds into more reactive CM bonds amenable to further functionalization is of fundamental importance in synthetic chemistry. We demonstrate here that the transformation of neutral bismuth compounds into their cationic analogues can be used as a strategy to facilitate CH activation reactions. In particular, the double CH activation of bismuth-‐bound diphenyl amide, (NPh2), is reported along with simple one-‐pot procedures for the functionalization of the activated positions. The organometallic products of the first and second CH activation steps were isolated in high yields. Analysis by NMR spectroscopy, single-‐crystal X-‐ray diffraction, and DFT calculations revealed unusual ground-‐state properties (e.g., ring strain, moderate heteroaromaticity), and provided mechanistic insight into the formation of these compounds.
