The reactivity of the Cyclopropyl cyanide in Titan’s atmosphere: a possible prebiotic mechanism
E. López, D. Ascenzi, P. Tosi, J. M. Bofill, J. de Andrés, M. Albertí, J. M. Lucas, A. Aguilar.
Phys. Chem. Chem. Phys., 20 (2018) 6198.
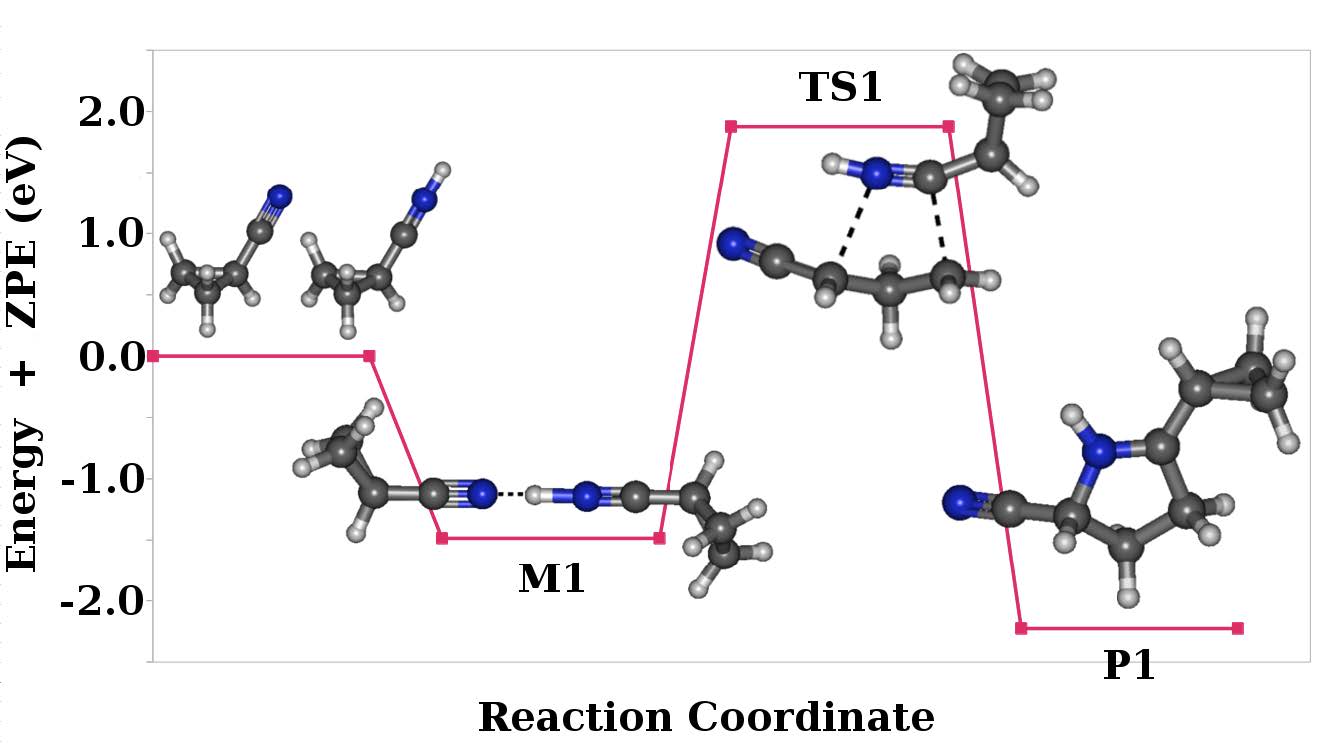
MP2 adiabatic potential energy profile showing the different stationary point (and structures) along the reaction pathway for the C4H5N + C4H5NH+ reactive process leading to the P1 covalent structure adduct on the ground singlet state of the system.
Cyclopropyl cyanide and other simple nitriles detected on Titan’s atmosphere could be precursors leading to the formation of organic macromolecules in its atmosphere. Reaction experiments done between cyclopropyl cyanide and its protonated form, in addition to the expected ion-molecule adduct stabilized by non-covalent long-range interactions, lead to another distinct species having the same mass to charge ratio (m/z) of 135 as is proved in this work. From a previous study of the neutral cyclopropyl cyanide potential energy surface (PES) which shows a partial biradical character it has been possible to characterize the formation through the bimolecular reaction of a new covalent cyclic organic molecule at the ab initio Möller-Plesset (MP2) level of theory, ensuring the connectivity of the stationary points by using the intrinsic reaction coordinate (IRC) procedure. Characterizing the reaction transition state, multireference calculations were done using a complete active space involving six electrons and six molecular orbitals [CAS (6 e-,6 m.o.)] This study opens the possibility of exploring the formation of new organic molecules by gaseous phase ion-molecule interaction schemes, such molecules having relevance in interstellar space and in astrobiology (and may be involved in the prebiotic molecular evolution).
