Ion-pair formation in neutral potassium-neutral pyrimidine collisions: electron transfer experiments
A. M. Mendes; B. Pamplona, S. Kumar; F. Ferreira da Silva, A. Aguilar, G. García, M. C. Bacchus_Montabonel, P. Limao-Vieira.
Front. Chem., 7 (2019) 157.
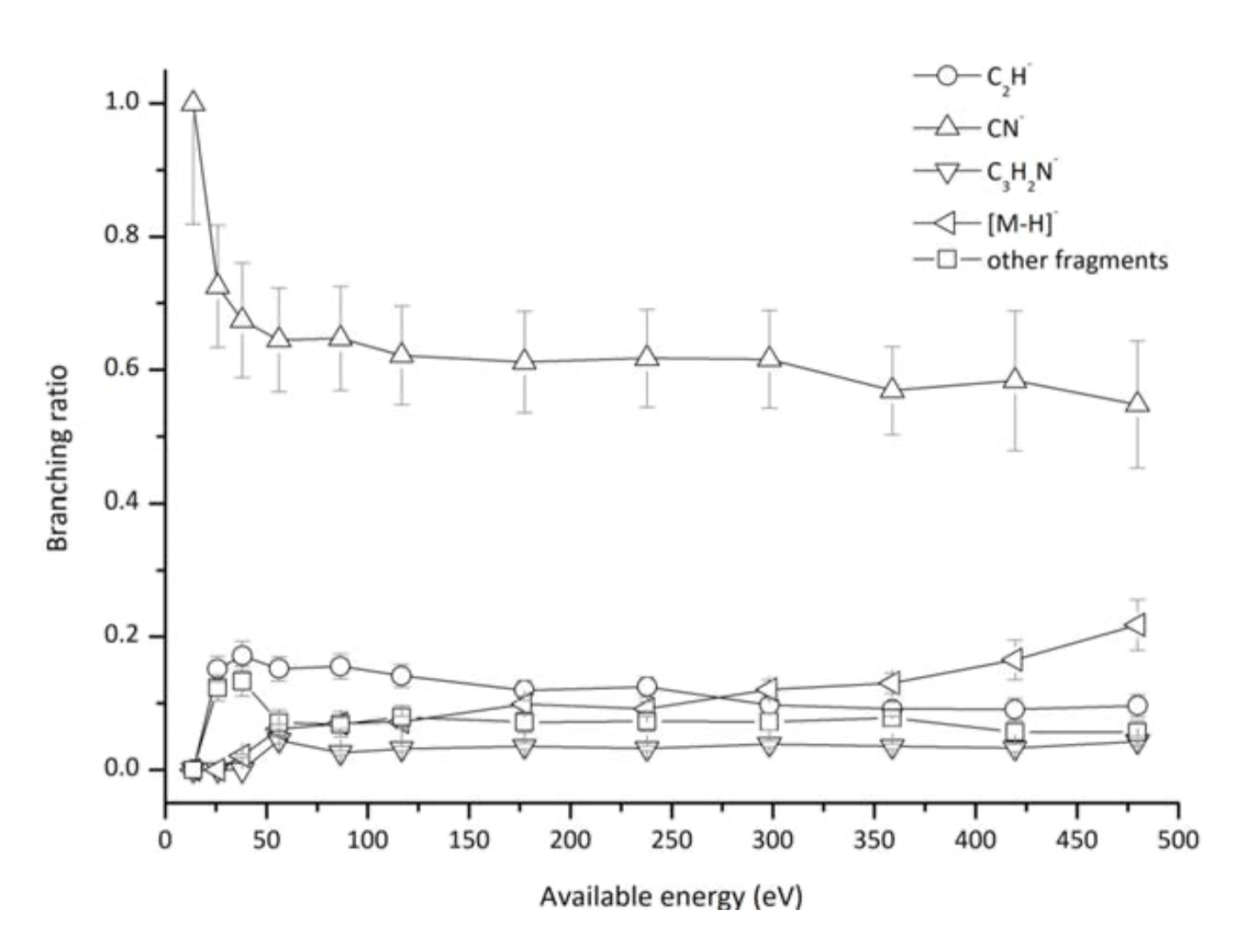
Pyrimidine branching ratios (fragment anion yield/total anion yield) of the main negative ions formed as a function of the collision energy in the center-of-mass frame.
We report on ion-pair formation in hyperthermal (30–800 eV) neutral potassium collisions with neutral pyrimidine molecules. Negative ions formed by electron transfer from the alkali atom to the target molecule were time-of-flight mass analysed and the fragmentation patterns and branching ratios obtained. The most abundant product anions have been assigned to CN− and C2H− and the electron transfer mechanisms are comprehensively discussed. Theoretical calculations were performed for pyrimidine in the presence of a potassium atom and provided a strong basis for the assignment of the lowest unoccupied molecular orbitals accessed in the collision process. In order to further our knowledge about the collision dynamics, potassium cation (K+) energy loss spectrum has been obtained and within this context, we also discuss the role of the accessible electronic states. A vertical electron affinity of (−5.69 ± 0.20) eV was obtained and may be assigned to a π*3(b1) state that leads to CN− formation.
