The gas phase oxidation of HCOOH by Cl and NH2 radicals. Proton coupled electron transfer versus hydrogen atom transfer
J. M. Anglada, R. Crehuet, A. Solé.
Mol. Phys., 117 (2019) 1430.
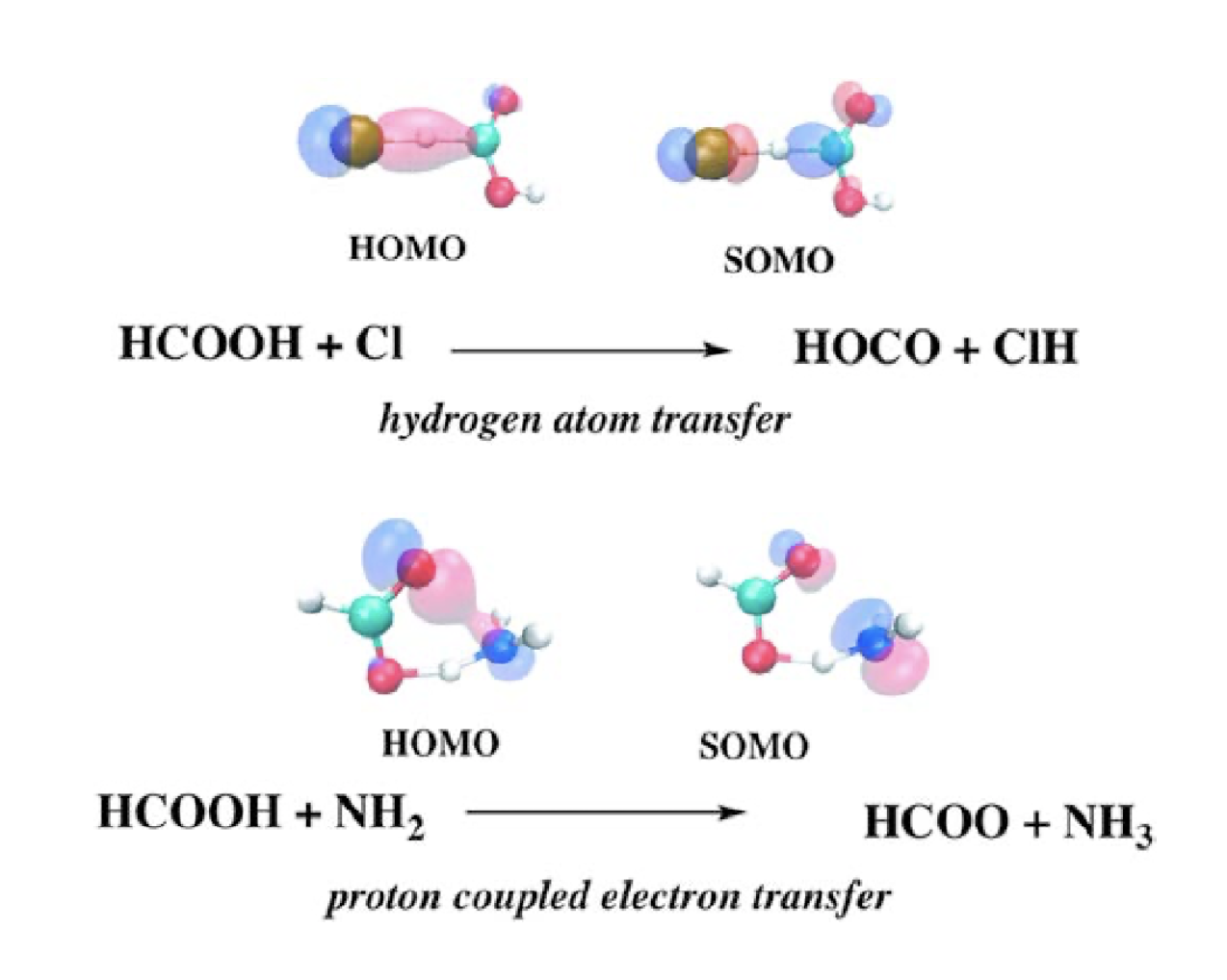
Examples of hat and pcet reaction mechanisms.
The reaction of formic acid (HCOOH) with chlorine atom and amidogen radical (NH2) have been investigated using high level theoretical methods such BH&HLYP, MP2, QCISD, and CCSD(T) with the 6–311+G(2df,2p), aug-cc-pVTZ, aug-cc-pVQZ and extrapolation to CBS basis sets. The abstraction of the acidic and formyl hydrogen atoms of the acid by the two radicals has been considered, and the different reactions proceed either by a proton coupled electron transfer (pcet) and hydrogen atom transfer (hat) mechanisms. Our calculated rate constant at 298 K for the reaction with Cl is 1.14 x 10−13 cm3 molecule−1 s−1 in good agreement with the experimental value 1.8±0.12/2.0 x 10−13 cm3 molecule−1 s−1 and the reaction proceeds exclusively by abstraction of the formyl hydrogen atom, via hat mechanism, producing HOCO+ClH. The calculated rate constant, at 298 K, for the reaction with NH2 is 1.71 x 10−15 cm3 molecule−1 s−1, and the reaction goes through the abstraction of the acidic hydrogen atom, via a pcet mechanism, leading to the formation of HCOO+NH3.
