A single point mutation converts GH84 O-GlcNAc hydrolases into phosphorylases. Experimental and theoretical evidence.
A. D. Tezé, J. Coines, L. Raich, V. Kalichuk, C. Solleux, C. Tellier, C. André-Miral, B. Svensson, C. Rovira.
J. Am. Chem. Soc., 142 (2020) 2120.
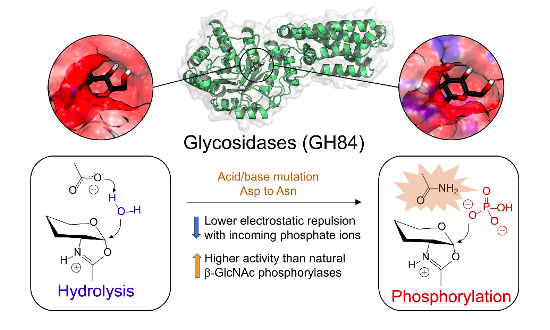
Glycoside hydrolases (GHs) and phosphorylases (GPs) are two major classes of enzymes responsible for the break down of carbohydrates in nature. Here we showed that two GHs (in particular, two O-glucosaminyl glucosidases) can be converted to efficient phosphorylases just by the replacement of an amino acid in the enzyme active site (Asp120Asn single point mutation). Using molecular dynamics and QM/MM metadynamics methods we show that the mutation changes the electrostatic potential at the active site and drastically reduces the energy barrier for phosphorolysis, which becomes favoured over hydrolysis. Noteworthy, kinetic experiments show that the mutated enzymes are over 10-fold more active than their corresponding naturally occurring phosphorylases. These insights expand our understanding of GH and GP mechanisms, providing a basis for the generation of enzymatic variants that could be amenable for the large scale production of valuable phosphorylated glycosides.
